In order to enhance the efficiency and lifetime of future battery systems, it is essential to understand the physical principles determining the functionality of a battery at the atomic scale.
With our expertise in the fields of solid-state physics and mechanics of materials, our research focuses on the coupling of mechanical and electrochemical processes, which occur in the different material components during loading and unloading of a battery.
Within the project DEFACTO, funded by the European Union, we apply atomistic simulations to study the effect of mechanical stresses, induced by intercalation and de-intercalation of Li ions in polycrystalline Li-Si anodes, on the electrochemical potential of Li, which characterizes the ionic diffusion and the electrical voltage.
Li-Si anodes are promising anode materials, since they provide high energy densities. However, concomitant large volume changes can induce mechanical degradation such as crack formation and loss of contact.
In order to reduce these kinds of unwanted, lifetime-limiting factors in cathode materials, in a project funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG), we explore which properties must exist within a crystal structure to ensure that the incorporation of Li ions leads to only negligible volume changes.
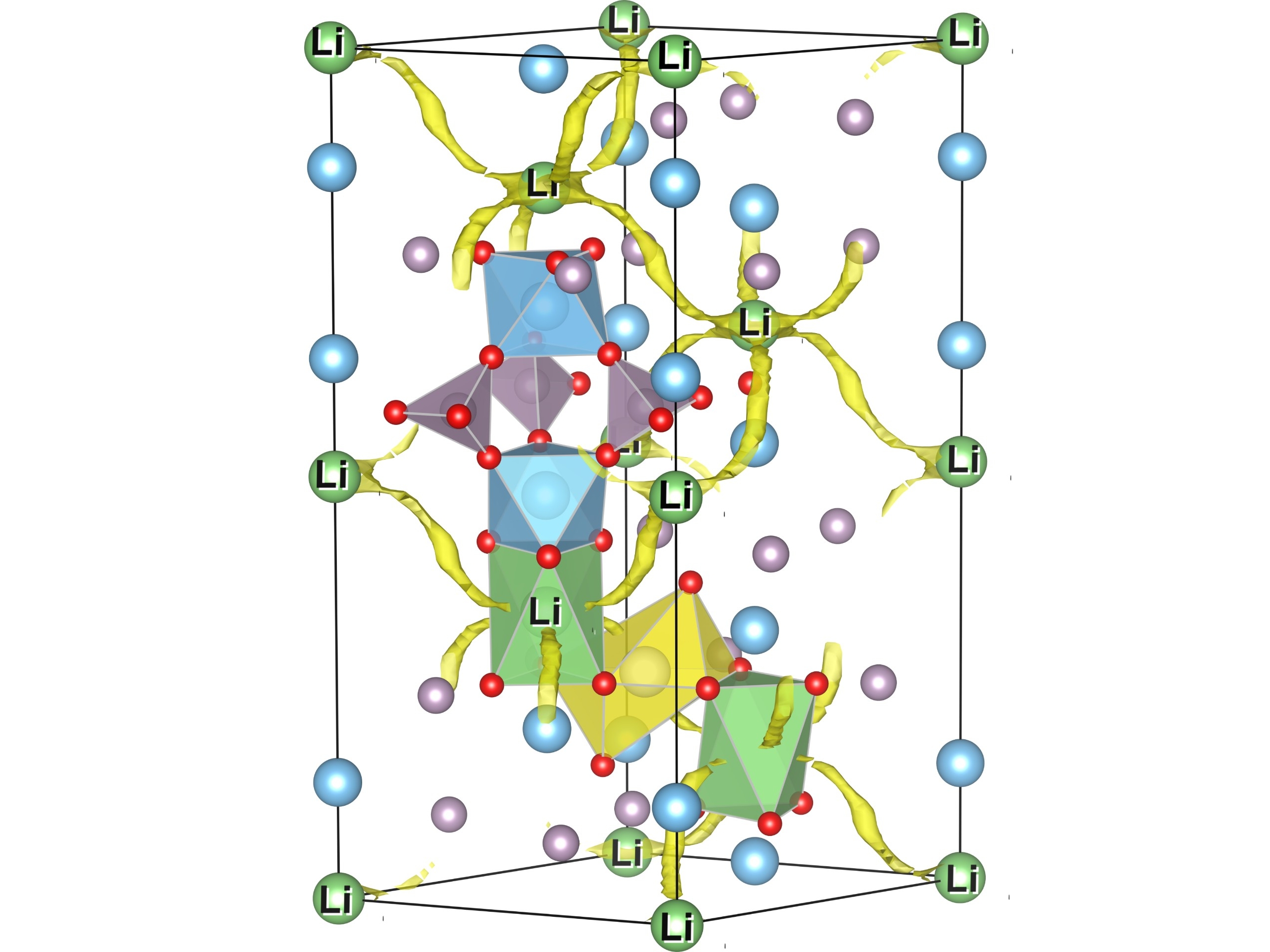
The use of ceramic materials as ion-conducting electrolytes in solid-state batteries has the potential to considerably enhance the safety, reduce the total weight and increase the energy density of novel battery cells as compared to conventional systems based on liquid electrolytes. By calculating characteristic properties of atomic migration paths, we provide information about the ionic conductivity of solid-state electrolytes for various elemental compositions.
An efficient, simulation-based high-throughput screening facilitates the determination of suitable combinations of elements and compounds, which saves resources, increases environmental compatibility, and reduces costs.
As a member of the Fraunhofer Battery Alliance, we closely collaborate with highly specialized partner institutes who apply experimental and computational methods to tackle numerous questions about the performance and development of materials and components of electrochemical storage devices.
 Fraunhofer Institute for Mechanics of Materials IWM
Fraunhofer Institute for Mechanics of Materials IWM